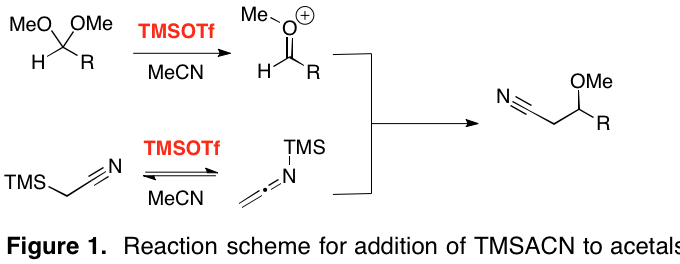
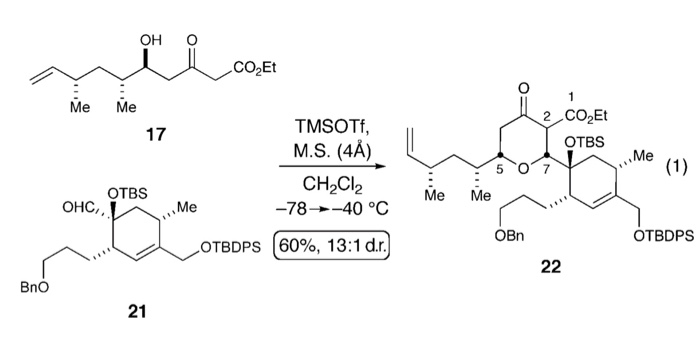
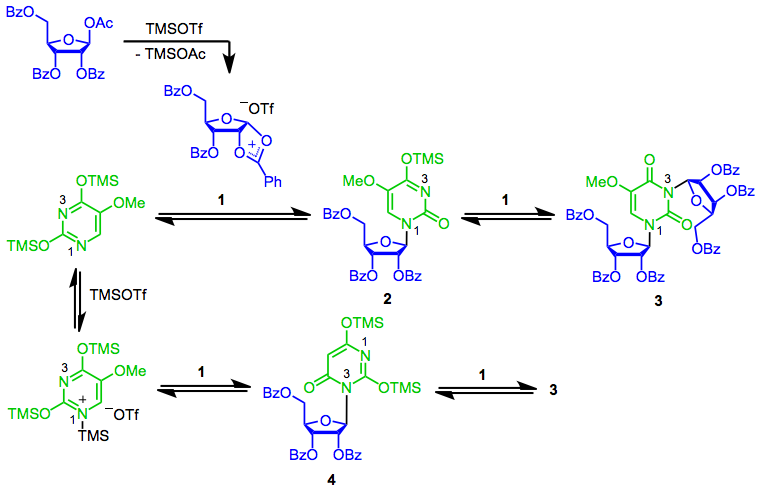
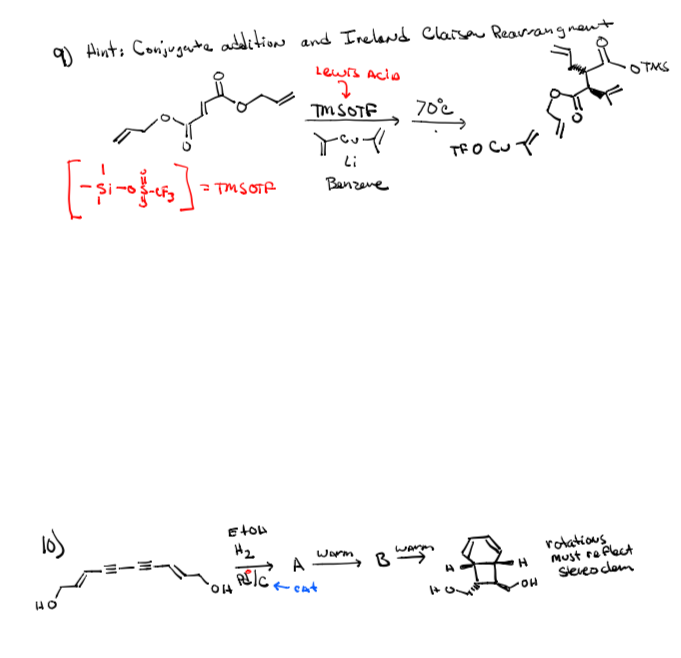
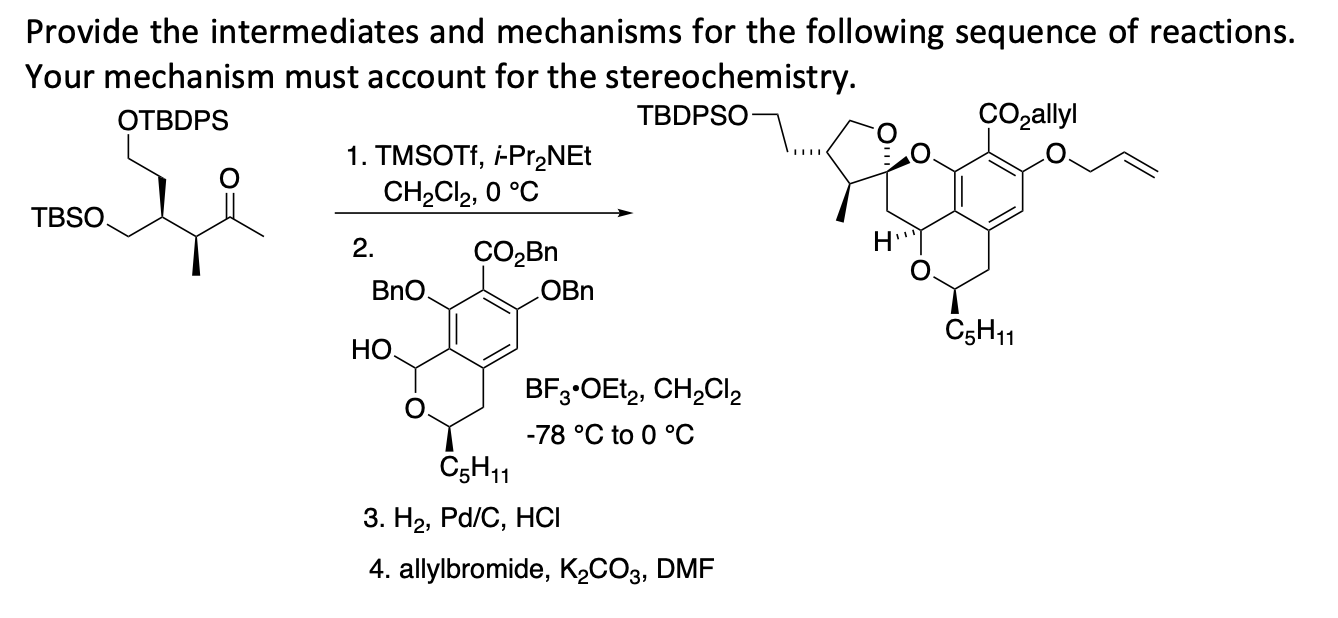
Mukaiyama addition of (trimethylsilyl)acetonitrile to dimethyl acetals mediated by trimethylsilyl trifluoromethanesulfonate - ScienceDirect

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram

TMSOTf‐Catalyzed Silylation: Streamlined Regioselective One‐Pot Protection and Acetylation of Carbohydrates - Joseph - 2012 - European Journal of Organic Chemistry - Wiley Online Library

Figure 1 from Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3. | Semantic Scholar
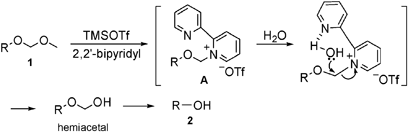
Remarkable effect of 2,2′-bipyridyl : mild and highly chemoselective deprotection of methoxymethyl (MOM) ethers in combination with TMSOTf (TESOTf)–2, ... - Chemical Communications (RSC Publishing) DOI:10.1039/B907910F

The reaction of acetal-type protective groups in combination with TMSOTf and 2,2′-bipyridyl; mild and chemoselective deprotection and direct conversion to other protective groups - ScienceDirect
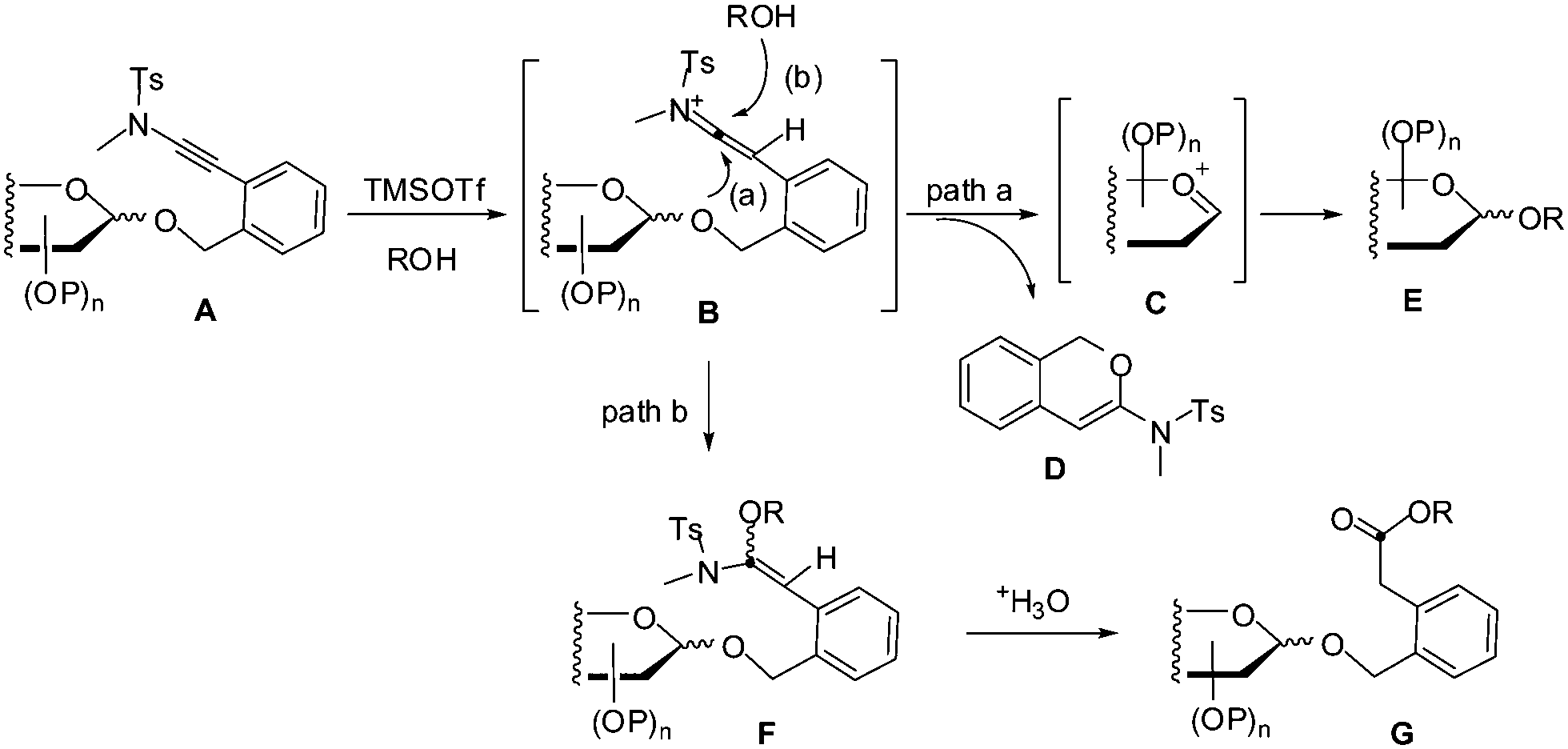
ortho -(Methyltosylaminoethynyl)benzyl glycosides as new glycosyl donors for latent-active glycosylation - Chemical Communications (RSC Publishing) DOI:10.1039/C5CC05651A

N‐Trifluoromethylthiosaccharin/TMSOTf: A New Mild Promoter System for Thioglycoside Activation - Carthy - 2019 - European Journal of Organic Chemistry - Wiley Online Library

Scheme 3 Proposed mechanism. Reagents and conditions: (i): 3 or 4 with... | Download Scientific Diagram

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram

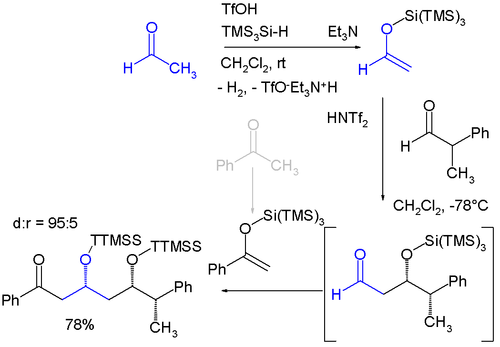
BF 3 ·OEt 2 and TMSOTf : A synergistic combination of Lewis acids - Chemical Communications (RSC Publishing) DOI:10.1039/B611333H

BF 3 ·OEt 2 and TMSOTf : A synergistic combination of Lewis acids - Chemical Communications (RSC Publishing) DOI:10.1039/B611333H
Scheme 3 Proposed mechanism. Reagents and conditions: (i): 3 or 4 with... | Download Scientific Diagram

Efficient activation of thioglycosides with N-(p-methylphenylthio)-ε-caprolactam-TMSOTf - ScienceDirect
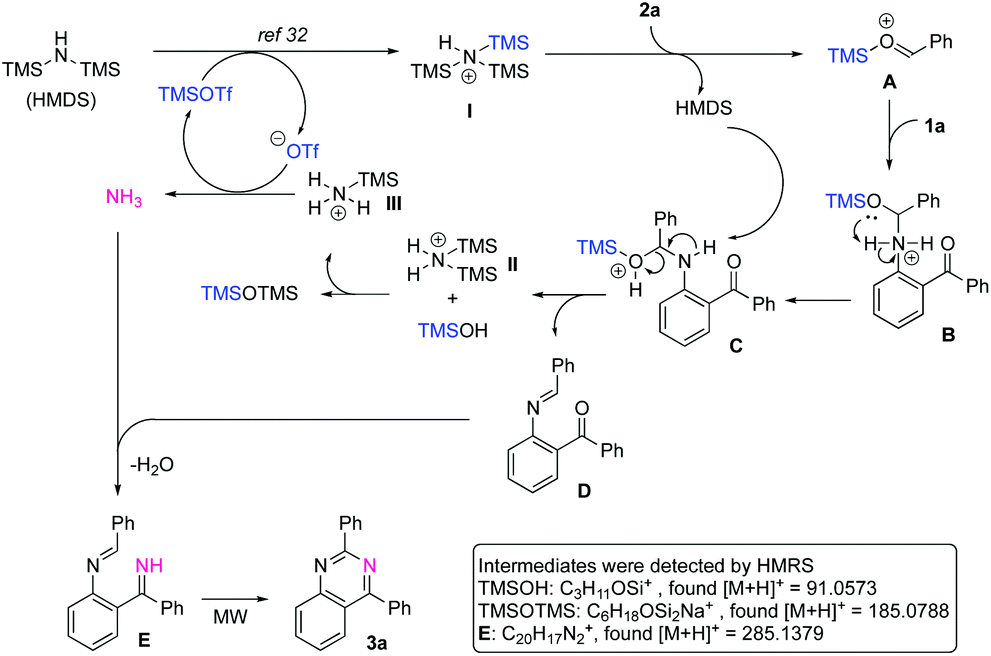
TMSOTf-catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as a nitrogen source under neat and microwave irradiation conditions ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB01507E
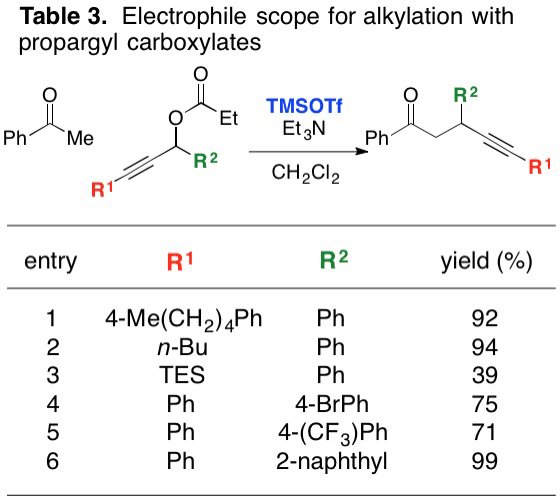
Report: New Reactivity Mediated by Trimethylsilyl Trifluoromethanesulfonate (62nd Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)

O‐Trifluoromethylation of N,N‐Disubstituted Hydroxylamines with Hypervalent Iodine Reagents - Matoušek - 2014 - European Journal of Organic Chemistry - Wiley Online Library

Tentative mechanism for the TMSOTf-mediated formation of dithioacetal... | Download Scientific Diagram